Enzyme that primes chromatin for DNA-repair has an on/off switch
A study led by Sebastian Deindl (Uppsala University/SciLifeLab) now demonstrates the mechanisms behind how a portion of the chromatin-remodeling enzyme ALC1 acts as an on/off switch for its enzymatic activity. In the long-term, these results could potentially open up new horizons for therapeutic intervention strategies in cancer.
Our genetic information is packaged into a highly compact state called chromatin. This packaging of DNA as chromatin regulates many vital processes that require access to the cell’s genetic material. ATP-dependent chromatin remodelers are enzymes specialized on altering the structure and packaging state of chromatin.
Principal Investigators Sebastian Deindl (Uppsala University/SciLifeLab) and Simon Boulton (Francis Crick Institute) together with their teams shed light on the molecular mechanisms that switch on and off the oncogenic chromatin remodeling enzyme ALC1 (short for Amplified in Liver Cancer 1).
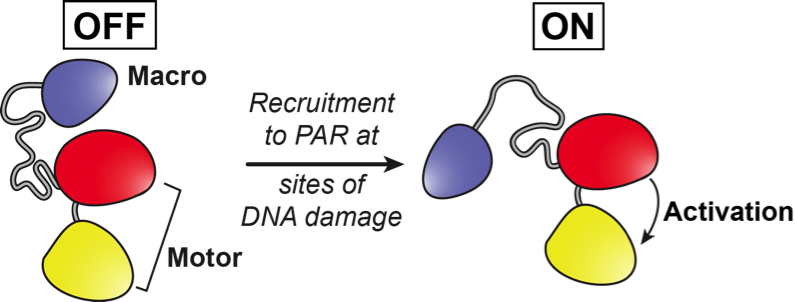
The business end of ALC1 is a motor domain that, just like in other chromatin remodeling enzymes, can hydrolyze ATP as fuel to move the enzyme along DNA and to change the packaging state of chromatin. “What is special about ALC1 is that next to the motor portion, this enzyme also possesses a so-called macro domain that can recognize PAR chains (poly-ADP-Ribose), a polymer that accumulates at sites where DNA has been damaged”, says Laura Lehmann, PhD student at Uppsala University and co-author of the paper.
By applying biophysical techniques to elucidate the overall shape of ALC1 and combining these approaches with cell-based and live-cell imaging experiments, the authors of the article in Molecular Cell dissect the molecular mechanisms how the remodeling activity of ALC1 is controlled by its macro domain.
“The macro domain of ALC1 is an additional portion of this chromatin remodeler that sits on the ATPase motor and shuts down its activity when it’s not needed. In a way, you can think of the macro domain as a ‘molecular brake’ that switches off the motor when there is no DNA damage. In the presence of DNA damage, the macro domain of ALC1 is bound to PAR chains that accumulate at the damage site. This recruits the remodeler to the site of action and at the same time shifts the shape of ALC1 in such a way that the brake is released from the motor. Activated ALC1 could then make the damaged DNA accessible for repair processes”, explains Sebastian Deindl.
These findings may explain how several mutations reported in human cancer can compromise the ‘off switch’ of ALC1. Indeed, when the authors introduced these cancer mutations into ALC1 in their experiments, they observed a ‘hyperactive’, permanently ‘on’ ATPase motor. “Such uncontrolled activity of ALC1 in the absence of DNA damage is expected to have severe consequences for the cell. Ultimately, a better molecular-level understanding of the regulatory mechanisms that control ALC1 activity may in the long term open up new horizons for therapeutic intervention strategies”, Deindl says.
Read a press release by Uppsala University
Watch a video about Sebastian Deindl’s research: