I am interested in understanding how neural hardware (neuron, synapse and connectivity patterns) shape network activity and thereby brain function. Specifically, we are exploring the following questions:
- What role do the network connectivity and network dynamics play in the transfer of information from one network to another?
- How neuronal and synapse properties interact with network structure to shape the network activity dynamics?
- How the activity dynamics of the network and information transfer can be controlled by external stimulation?
In a way we use the ‘static’ data about the brain — in the form of neuron properties, synapse properties (which are determined largely by gene expressions) — and convert it to dynamic data.
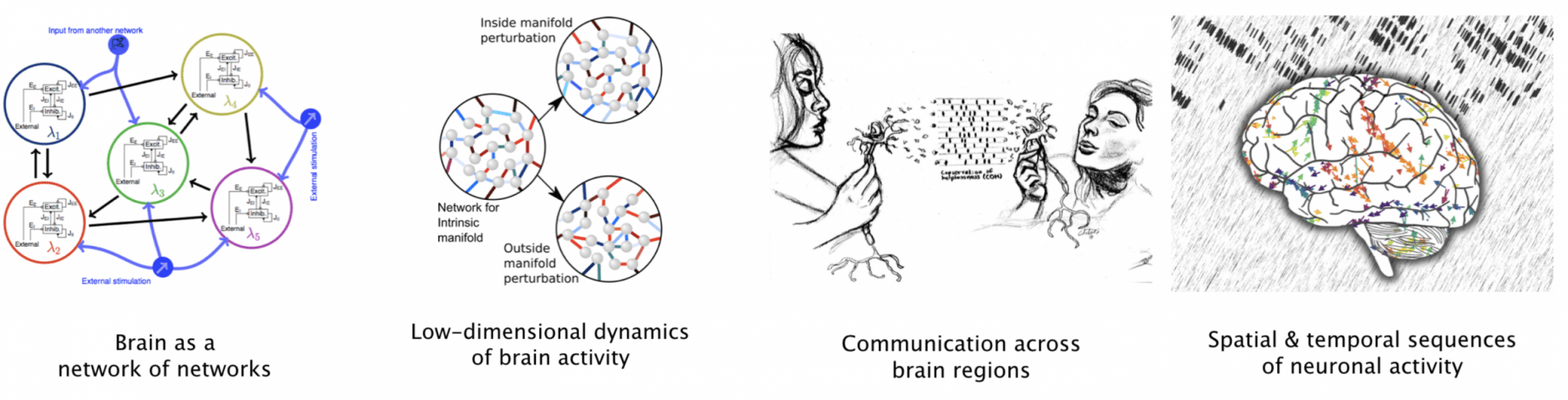
We use analytical methods from statistical mechanics, probability theory, graph theory and control systems theory, and combine them with numerical simulations of large-scale neuronal networks of different brain regions.
One of the goals of our research is to develop mathematical models of brain diseases and create a theoretical framework to understand the mechanisms underlying the emergence of disease related aberrant activity dynamics in diseases (e.g. Parkinson’s diseases, epilepsy, anxiety). Eventually such a quantitative understanding of brain diseases would pave the way for the development of novel brain stimulation protocols to control or correct the disease-related brain activity. Therefore, we are neuralizing the control system theory and developing tools to control the dynamics of neuronal networks by external stimulations methods.
Group Members
Archishman Biswas | PhD Student
Satarupa Chakrabarti | Postdoc
Movitz Lenninger | Postdoc
Hauke Wernecke | PhD Student
Moritz Pesl | Visiting internship student