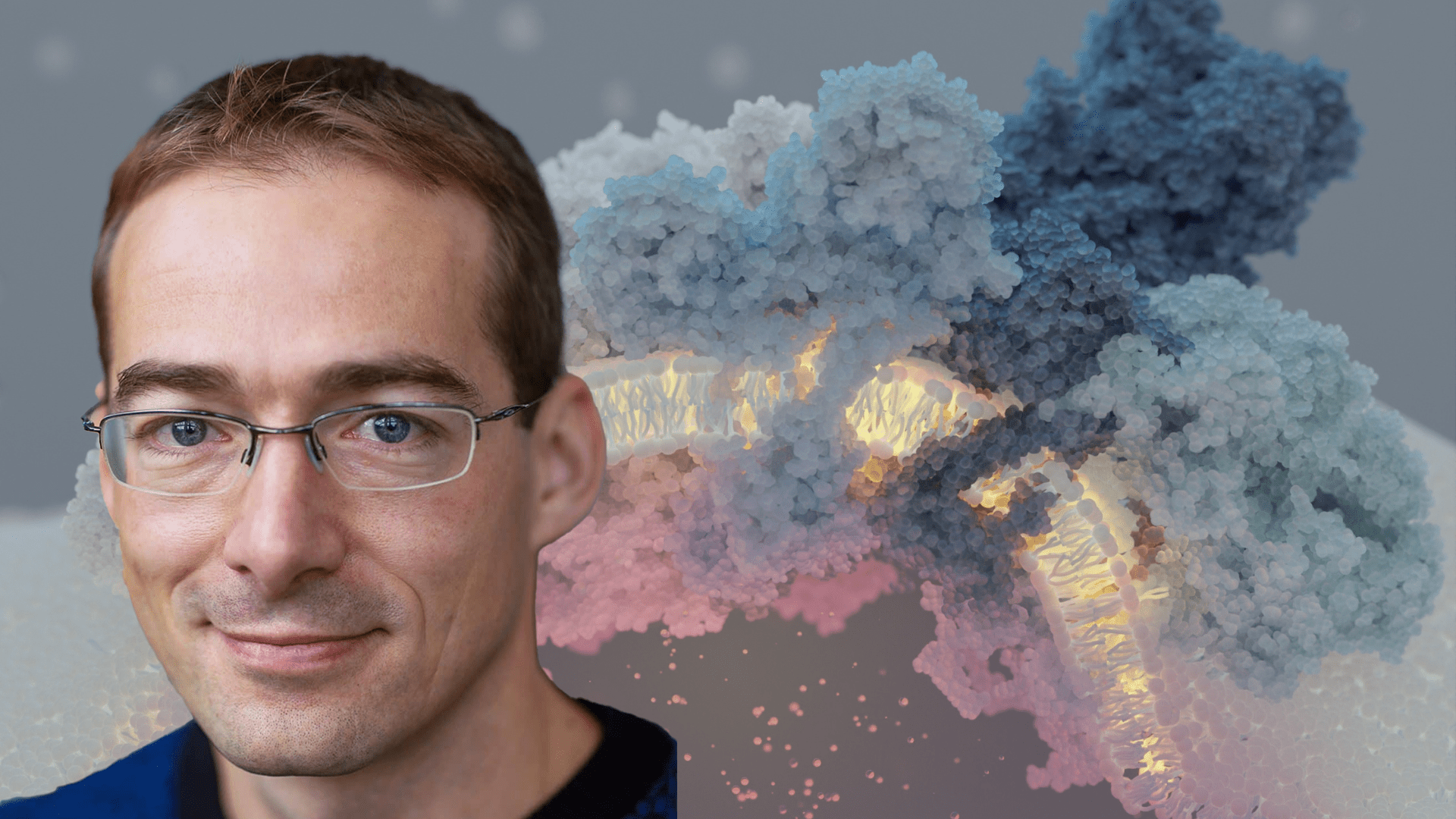
A massive supercomplex induces membrane curvature for cellular respiration
A study led by SciLifeLab researcher Alexey Amunts has discovered a group of 150 proteins in the mitochondria of a single-celled organism that helps shape the cell’s power-producing structures for more efficient energy conversion. This finding gives us new insights into how these protein groups improve the production of ATP, the body’s energy source.
Mitochondria are organelles within our cells that produce energy through a process called cellular respiration. This energy production relies on a series of reactions that happen in the inner membrane of mitochondria.
The study, published in Nature, found that all four complexes of proteins responsible for these reactions are connected into a supercomplex in a single-celled organism Tetrahymena thermophila living in lakes. By using advanced imaging techniques and simulations, Amunts and his team described that the supercomplex is made of 150 proteins, with hundreds of lipid molecules and structures that span the membrane.
This massive protein group actively helps shape the inner membrane of the mitochondria, making it more curved. This change in shape improves the production of ATP, the molecule that provides energy for the cell.
An exciting aspect of the study is the discovery of how a protein in one of the reaction groups is split and extended to create connections between different protein groups. This shows how nature can utilize molecular structure changes to improve cooperativity on the molecular level.
This research highlights the role of protein evolution in forming supercomplexes that not only help with energy conversion reactions but also actively shape the inner membrane of mitochondria. This dual function supports cellular respiration and provides the fuel necessary for life.
This study is funded by the ERC, Knut and Alice Wallenberg Foundation and the SSF Future Leaders program.
Publication: ”Structural basis of mitochondrial membrane bending by the I–II–III2–IV2 supercomplex.” Nature, volume 615, pages 934–938 (2023) https://www.nature.com/articles/s41586-023-05817-y