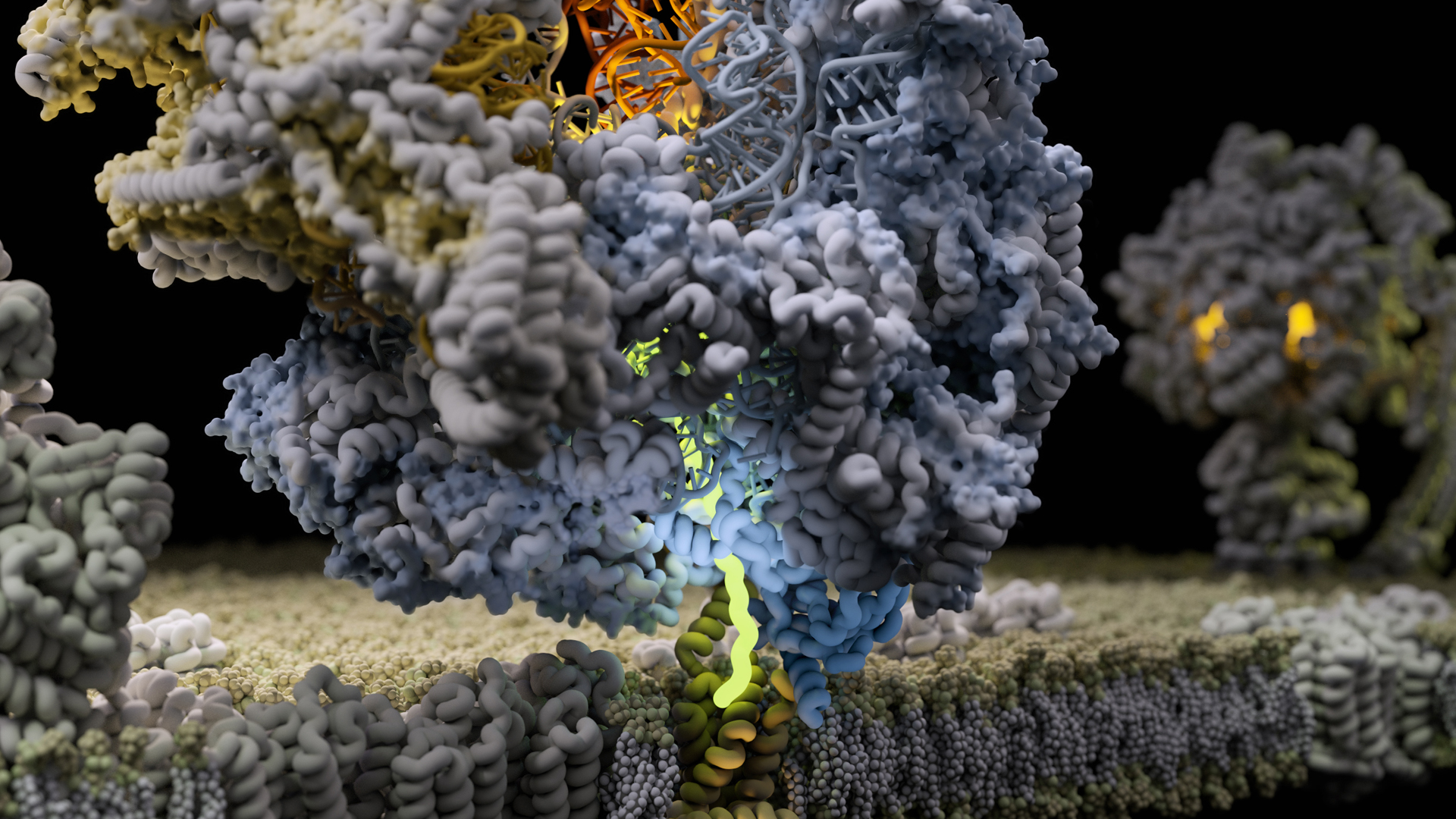
Cryo-EM reveals how energy-producing complexes are formed inside the mitochondria
SciLifeLab Fellow Alexey Amunts’ group has, together with an international team of scientists, been able to explain the process in which mitoribosomes synthesize and tether the specialized transmembrane proteins, vital for the mitochondrial electron transport chain, by using cryo-EM. The results were published in Science and the study was featured on the front cover.
Oxygen from the air we breathe and the food we eat provides fuel for the mitochondrial power plants located in the cytoplasm. The energy production itself occurs in bioenergetic “nano-factories” incorporated in specialized mitochondrial membranes. Inside these nano-factories, ions and electrons are cooperatively transported to generate chemical energy. In order to maintain the mitochondria and to duplicate its complex machinery during cell division, mitochondria specific ribosomes, called mitoribosomes, are used.
“Seven years ago, our work on the structure of yeast mitoribosomes was termed the Resolution Revolution. The current study represents an additional advance on the original breakthrough. Not only does it reveal how the human mitoribosome is designed at an unprecedented level of detail, but it also explains the molecular mechanism that drives the process of bioenergetics to fuel life”, says SciLifeLab Fellow and last author, Alexey Amunts (Stockholm University), in a press release press release from Stockholm University.
Since molecular machines rely on the flexibility of their subunits and internal conformal changes to operate. The team had to use advanced equipment to collect and process 30 times more data than previously, enabling them to obtain enough information to describe the conformational changes during the process of protein synthesis and association with the membrane adaptor.
“Our study reveals that the mitoribosome is much more flexible and active than previously thought. The discovery of intrinsic conformational changes represents a gating mechanism of the mitoribosome without similarity in bacterial and cytosolic systems. Together, the data offer a molecular insight into how bioenergetic proteins are synthesized in human mitochondria,” adds Alexey Amunts.
The discovery of the mitoribosomal gating mechanism represents a big step forward and the structural data provides the scientific community with a long awaited understanding of how bioenergetic proteins are synthesized by the mitoribosomes. This knowledge can be used to develop new therapeutics as well as provide insight in human diseases.
Read the entire press release here.