Researching cells through colorful imaging
The Cell Profiling unit at SciLifeLab has a unique library of more than 25 000 validated antibodies, which are used to create strikingly colorful images describing the inner workings of cells. But how does the image creation work exactly, what does Head of Unit Charlotte Stadler see for the future of Cell Profiling and how can you get access to the technologies in your project?
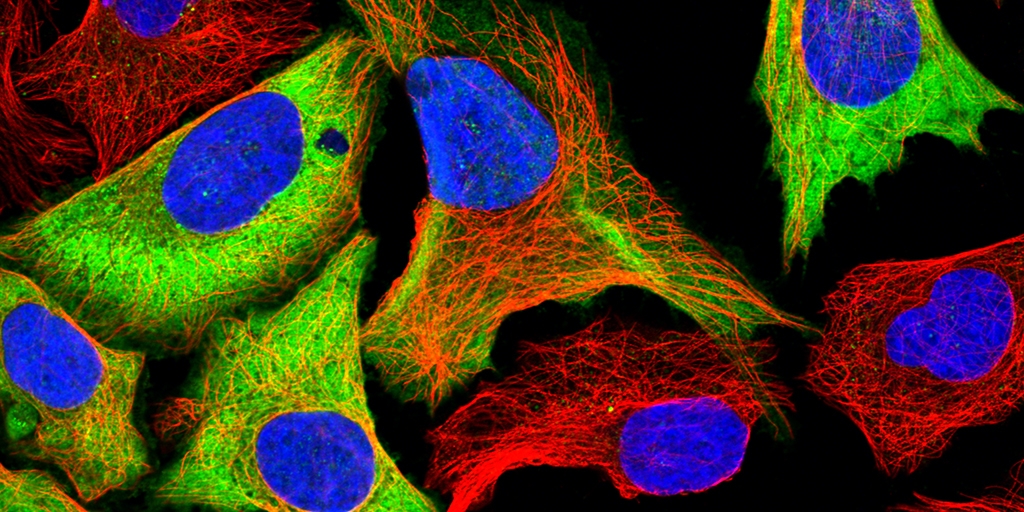
In essence, the Cell Profiling unit grows and treats cells, adds antibodies with fluorescent dyes attached to them, takes pictures of this and looks at it in a microscope – a process that gives them images in smashing colors, describing where proteins and other structures are located in individual cells.
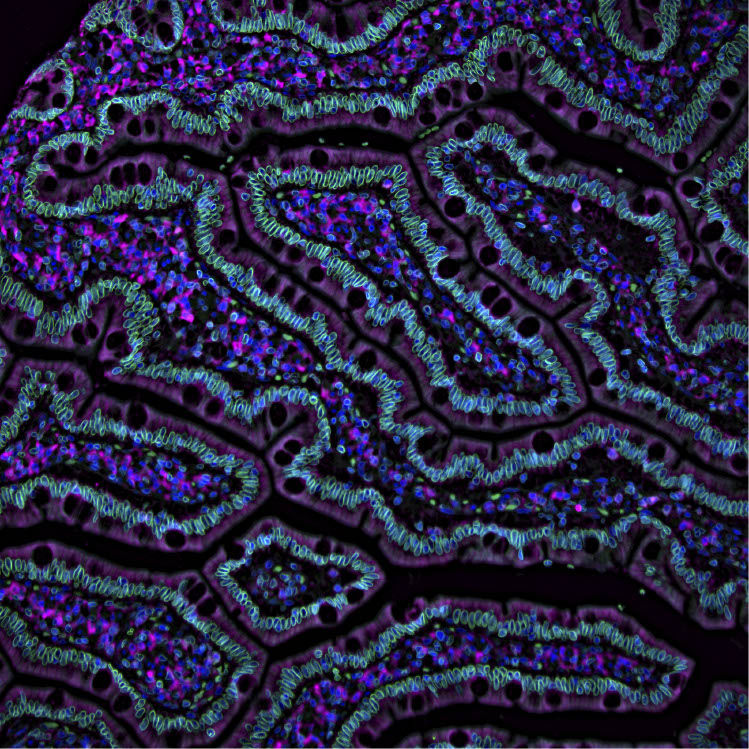
Why antibodies are used when taking pictures of proteins? Essentially because they are really good at finding specific things. If a protein is to be analyzed, certain antibodies know where to find it in the cell and attach to it. When preparing the antibodies with a fluorescent dye, the proteins they attach to can be viewed in a microscope capable of visualizing these fluorescent colors. Different fluorophores, molecules that can emit light, are used to create images of different colors, where different parts of the cell glow with different colored light. In this way, you can see the boundaries of a cell in a certain color and other structures in other colors, making strikingly pedagogical works of art.
A unique library
The Cell Profiling unit has got access to more than 25 000 validated antibodies from the Human Protein Atlas project, a project that started in 2003 with the aim of mapping all human proteins in cells and tissues. In practice, having access to the library means that researchers can use the unit to visualize a lot of different proteins – rather than having to analyze a single protein due to limited resources. When using Cell Profiling, you do not need to purchase a whole vial with a hundred microliters of each specific antibody, which is often the case if you buy antibodies from a commercial company. You only need to pay for the amount of antibody you use, which reduces the cost per protein to look at. The unit also helps with preparing the samples and taking the images.

It is easy to think in terms of your perceived reality. When the only option is to analyze one or a few proteins due to lack of resources, there is not much of a reason to think of research projects that include hundreds. Having access to a whole library of antibodies allows researchers to explore new ideas.
“The antibody library is a truly unique resource, and we believe that many researchers are not aware of these possibilities. My hope is that researchers would come to us wanting to use hundreds of antibodies from our library to answer complex questions”, says Charlotte Stadler.
The Human Protein Atlas project started in 2003 with the aim to map all proteins in cells and tissues. So, why would you need to analyze more than this? The atlas has mapped the proteins in a standardized manner, during normal circumstances. Further research and units such as Cell Profiling is needed when there is a need to investigate situations out of the ordinary.
“In contrast to the Human Protein Atlas, we make tailor-made experiments where we characterize cells under particular circumstances, how they react when a substance is introduced for example”, says Charlotte Stadler.

She explains the difference by comparing the cell to a company. The Human Protein Atlas investigates the employees of the company – the proteins – on a regular day, while the Cell Profiling unit can examine the company when there is a financial crisis, to see whether the dynamic between the employees differ during the more stressful environment.
Grown up at SciLifeLab
Charlotte Stadler is truly passionate about method development. This is also the type of research she takes on as Head of Unit, 20 percent of her work can be dedicated to research on developing the methods they use at the unit. She is excited about a new multiplex instrument – called CODEX, that can analyze more than 25 proteins at a time in the same sample, but she is also intrigued by improving tissue analysis.

Another area Charlotte Stadler has recently studied is sample preparation of non-adherent cells. Adherent cells are cells that prefer to grow on things, such as skin cells. In contrast, non-adherent cells are often on the move, like blood cells that circulate in the bloodstream. Because of this, it’s hard to get them to stick to surfaces – such as microscope slides, the cells are easily washed away during sample preparation. Charlotte Stadler and the Cell Profiling unit have now optimized a protocol to be able to analyze non-adherent cells with greater success.
“This is a typical example of internal method development where focus lies on improving the technologies and methods needed to answer the biological questions the users may have”, says Charlotte Stadler.
One could almost say that Charlotte Stadler has grown up at SciLifeLab. She worked with the Human Protein Atlas project as part of her master’s thesis, and stayed at the project for her PhD, developing and integrating many of the strategies used for antibody validation within the Human Protein Atlas today. After her PhD she was offered a job at the project. This is also why she had a bit of an identity crisis before she started as Head of Unit. Should you really work at the same place for your entire career, she asked herself. She explained the situation to her supervisor and Head of Unit at the time, Dr Emma Lundberg, who responded with offering her own job to Charlotte Stadler – a job that came with new challenges, more strategic thinking and lots of responsibility. The answer to the question was in the end quite easy: If you like it, of course you should stay.
“As long as you feel that you are doing something meaningful and you are motivated to do a good job, there’s no reason to change just for the sake of changing. The Human Protein Atlas will always be close to my heart and I’m lucky to still work in close collaboration with the Human Protein Cell Atlas team”, says Charlotte Stadler.
Using the unit
The Cell Profiling unit helps researchers, or users as they are called, answer biological questions.
What could a researcher expect, timewise?
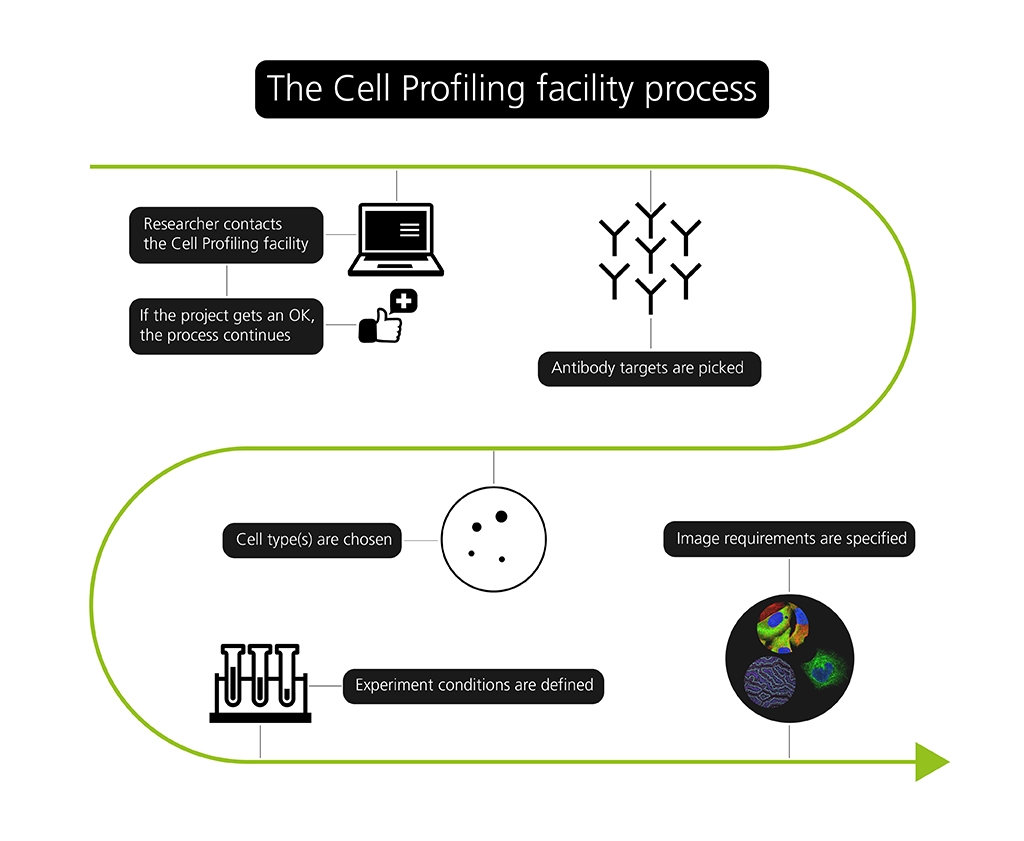
“Usually, the process from first contact to project start takes about a month, depending on how well prepared the applicants are, the more clear their idea is, the faster we can define the project and start analyzing”, says Charlotte Stadler.
The process of using the Cell Profiling unit seems to be quite informal. The best way to start a project is by sending an email to the unit. A meeting is usually set up after the email, where the researcher and Charlotte Stadler discuss the project, to see whether it is a project the unit can contribute to – and to set everyone’s expectations straight. After the meeting, the potential user is asked to submit a more formal application, which Charlotte Stadler assures “is not as complicated as it sounds”.
A standard screen, as they call it, consists of growing cells, adding these cells to plates that usually take 96 samples each, coloring the samples with antibodies relevant to the specific project and taking images with the microscopes. This takes two to three weeks, depending on whether the images can be taken automatically or not.
“If we need to look at proteins that are located in very specific structures or during rare events, we may need to image them manually one by one to capture what we are looking for, and this takes a little longer”, says Charlotte Stadler.
What can’t you help out with?
“We don’t look at living cells. We don’t do more complex image analysis either, other units can help with that. We usually do not take on projects that only need help with microscopy and have their own samples prepared. What makes Cell Profiling unique is our antibody library and our experience and robust infrastructure in sample preparation that is shared with the Cell Atlas team of the Human Protein Atlas. But we accept most projects that need help with the process from sample prep to microscopy and that preferably need to use a lot of different antibodies, that’s our specialty and what makes us unique”, says Charlotte Stadler.
One project they recently helped out with was a study where the researchers wanted to investigate a hundred different proteins in a small structure inside the cell, called the nucleolus. They wanted to see what happened when the cell was treated with a particular substance – a toxic peptide, and whether a few specific proteins were affected or if the structure as a whole was. They knew beforehand that something happened, but not exactly what. According to Charlotte Stadler, this is a good example of a project where the unit’s expertise and vast antibody library can be of use.
The future of Cell Profiling
Tissue analysis is often more complex than analyzing cells grown in a lab. Currently, the Cell Profiling unit only do limited numbers of tissue based projects as they are lacking the infrastructure needed to do tissue work at large scale, something Charlotte Stadler aims to change.
“My vision is for the unit to analyze proteins in tissue and move closer to preclinical research and the healthcare sector. In line with this comes the new highly multiplex technologies that we are currently implementing and hope to start offer later this year”, says Charlotte Stadler.

A new instrument called CODEX, developed by Akoya Biosciences, can look at multiple proteins at the same time – around 25 instead of four to five as in Cell Profiling’s current technologies. The unit have been one out of ten carefully selected labs world wide to be part of the early access program for the CODEX technology, which is now released for a broader market. They thus have a head start with the new technique, but still holds off with offering the service to researchers. They want to make sure that it delivers robust and credible data before opening up for submissions. When up and running, this technique may lead to more complex studies.
“Take tumors for example. They can be very heterogeneous, which makes it hard to determine how groups of cells differ from, and interact with, each other when you only look at one protein at a time. Analyzing many at the same time makes this a lot easier. These new highly multiplex technologies open up for new discoveries to be made in a much more efficient way, and I think this is the new era in tissue pathology”, says Charlotte Stadler.
Through an EU funded project called EPIC XS, researchers can now apply to perform projects at the Cell Profiling unit – free of charge. The first call for proposals is opening up shortly, and new calls will then follow every 3 months. More information can be found here: https://epic-xs.eu/, or, if preferred, the unit can be contacted directly at: cellpro.unit@localhost
Text & Photo: Niklas Norberg Wirtén





