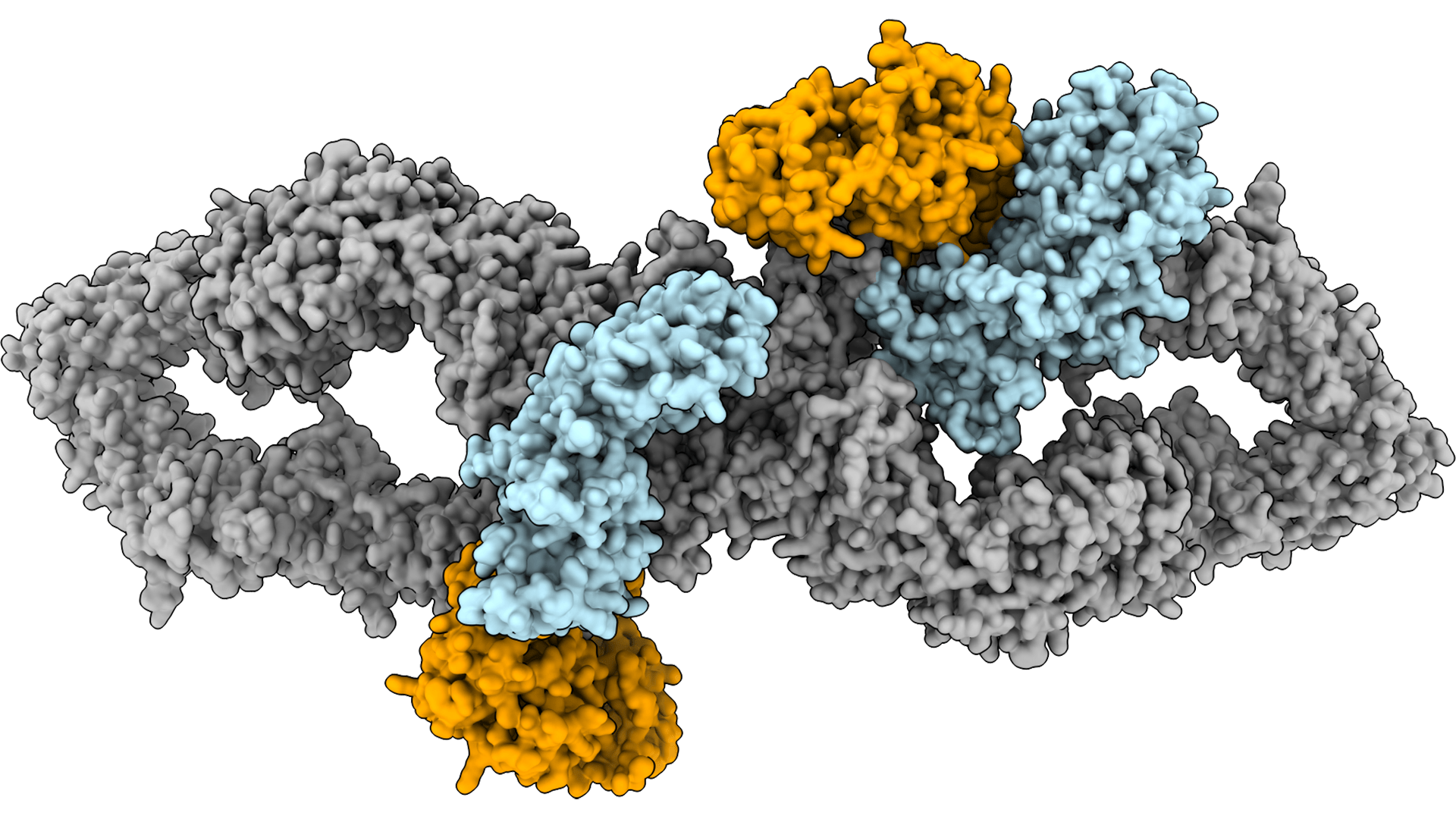
SciLifeLab researchers decipher the structure of tumor suppressor neurofibromin
Three SciLifeLab Cryo-EM researchers from Stockholm University, Andreas Naschberger, Rozbeh Baradaran and Marta Carroni, have, in collaboration with Bernhard Rupp at the Institute for Genetic Epidemiology in Innsbruck (Austria), determined the first high-resolution structure of the human protein tumor suppressor neurofibromin, by using single-particle cryo-EM.
Neurofibromin mutations are known to be the cause of the hereditary monogenetic disease neurofibromatosis type 1, one of the most common hereditary diseases, with a birth incidence of 1 in 3000. People suffering from neurofibromatosis type 1 are affected by a wide variety of symptoms related to the insurgence of benign or malignant tumors in the skin and nervous system. Neurofibromin has also been shown to be mutated in 5-10 percent of all spontaneously occurring tumors. Since neurofibromin is a regulator of the proto-oncogene Ras, it has also become a focus of cancer research.
The results from the Cryo-EM study, published in Nature, reveals that neurofibromin is a clever piece of cellular engineering, harboring two functional domains necessary for Ras-regulation, the membrane-anchoring domain Sec14-PH and the GTP-ase activating domain GRD. These domains undergo large conformational movements in order to downregulate Ras. Understanding how Ras is downregulated by neurofibromin is crucial for the development of new cancer treatments and the new study has contributed to the first steps taken towards detailed molecular studies of Ras-regulation.
“The collaboration among this “quartet” (Naschberger, Baradaran, Rupp and Carroni) is a very lively and fruitful one and it is nice to unite our scientific and technical knowledge towards the understanding of neurofibromin”, says Marta Carroni (SU), Head of the cryo-EM Unit at SciLifeLab Solna.
“This work paves the way to a number of studies that can now continue to unravel the fine mechanisms of neurofibromin, its interaction with other molecular partners and involvement in cancer”, continues Andreas Naschberger (SU), who initially conceived the study during his first postdoctoral studies in Bernhard Rupp’s laboratory.
“Additionally, this work is a good example of a spontaneous collaboration in science corridors, giving birth to exciting results”, adds Rozbeh Baradaran (SU), now a research fellow at MRC-LMB in Cambridge, UK.
The importance of technique itself cannot be understated.
“It is amazing how structural biology and thus the entire biological and biomedical research has changed and will continue to change, through cryo-EM. With the most modern instruments and new detectors, data can be recorded overnight and often processed in a few days”, says Bernhard Rupp (The Institute for Genetic Epidemiology in Innsbruck), corresponding author, and world-known crystallographer.
The Cryo-EM unit at SciLifeLab has received generous donations from The Knut and Alice Wallenberg Foundation (KAW) during the last five years, making it possible for many researchers around the country and in the Nordics to study the molecular mechanisms of important cellular players, such as neurofibromin
Watch a movie of neurofibromin here