Shedding light on the Amyloidogenic Pathway, involved in Alzheimer’s disease
Facilitated by the SciLifeLab Advanced Light Microscopy unit (ALM), researchers from Karolinska Institutet have been able to solve some of the mysteries within the amyloidogenic pathway, which is involved in the development of Alzheimer’s disease.
Alzheimer’s disease (AD) is a neurodegenerative disorder that is responsible for around 60–80% of all cases of dementia. The typical life expectancy following diagnosis is three to nine years. Since effective drugs are still lacking, the disease puts huge social and economic burden on the society. Extracellular amyloid plaques, neurofibrillary tangles, and loss of neuronal connections in the brain are the fundamental causes of the disease but the underlying mechanisms are still not completely understood.
So far, scientists know that the amyloid precursor protein (APP) is responsible for the production of amyloid β-peptides (Aβ) of variable lengths in neurons. The variant containing 42 amino acids (Aβ42) is directly responsible for the buildup of Aβ plaques in the brain, making it a key player in the development of AD.
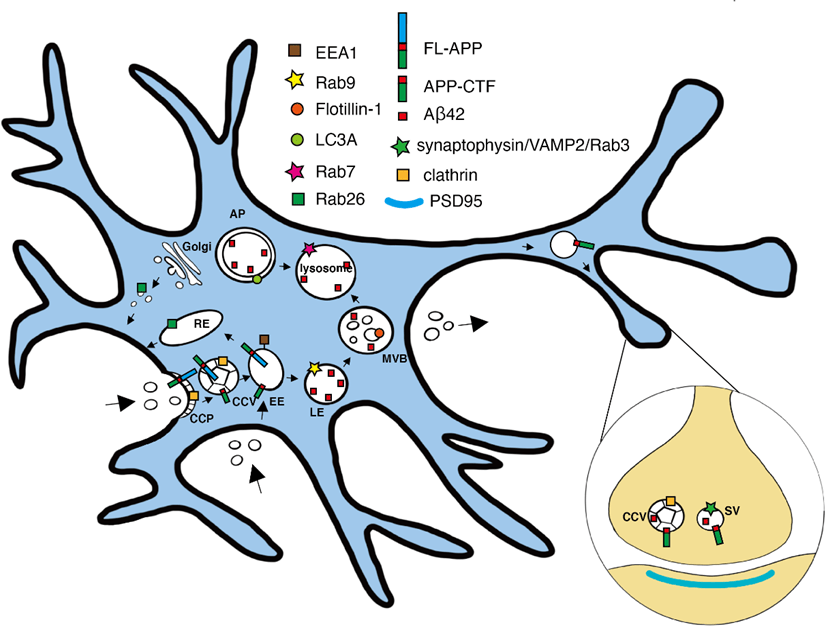
Facilitated by the SciLifeLab Advance Light Microscopy unit (ALM), Yang Yu, Lars Tjernberg and Sophia Schedin-Weiss (Karolinska Institutet) set out to clarify the subcellular locations of the fragments involved in the amyloidogenic pathway in primary neurons with a focus on Aβ42 and its immediate substrate, APP C-terminal fragment (APP-CTF).
The researchers used mouse primary hippocampal neurons that were immunolabeled and imaged by stimulated emission depletion (STED) microscopy, including three-dimensional three-channel imaging. This enabled creation of a model of how APP-CTF and APP N-terminal fragments (APP-NTF) are released in vesicles budding from early endosomes in soma.
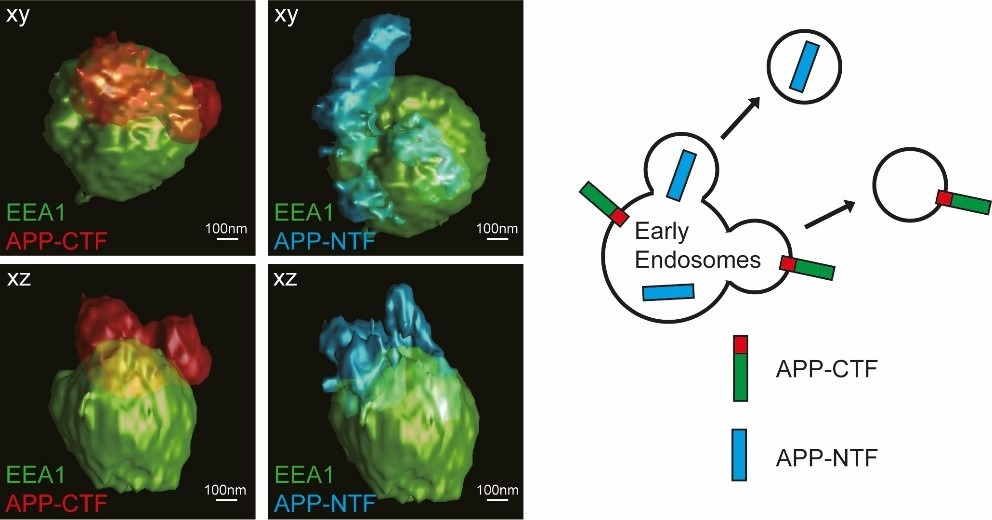
The results, published in the Journal of Alzheimer’s disease, helped the authors paint a picture of where the different cleavages of APP took place and where the different fragments ended up. The findings provide a super-resolved insight into amyloidogenic APP processing in primary neurons and might be vital for the development of any future Alzheimer’s disease treatments.
“We are very grateful for the possibilities super-resolution microscopy can offer. Unraveling the nano-level organization of the amyloidogenic APP processing in neurons is a big step towards understanding Alzheimer disease”.