Side effects: To prevent or not to prevent
Mia Wadelius and Pär Hallberg, both chief physicians at Uppsala University, have spent a lifetime trying to understand side effects of drugs – with research on the swine flu vaccine Pandemrix being their most recent published work. It turns out that implementing their findings in health care is easier said than done, however.
When treating patients with a drug, not everyone will respond to it in the same way. Most of them will hopefully get the desired effect. A few will, however, gain no effect at all and others will have negative experiences of the medicine – some severe, some less so. Weight increase from anti-psychosis drugs, fractures from medicine treating bone fragility, narcolepsy from the Pandemrix vaccine – the list of unwanted side effects is long.
Sometimes you have to accept the side effect because there is simply no alternative. Take cancer for example. The unwanted effects of the treatment can be massive, but it is often the only chance there is to recover.

Side effects can, however, also lead to unnecessary suffering and less than optimal treatment – therapy may have to be stopped completely if the reactions are too severe, and in many cases there are other drugs available. It is these side effects the Swedegene project aims to explain, and remedy, through genomics.
So, how do you explain why some patients experience side effects with the help of genetics? Swedegene does it, simply put, by analyzing DNA and trying to find out if the DNA of those affected are different from those who are not – or in science lingo: They look for genetic markers.
Smitten by side effects
Mia Wadelius and Pär Hallberg have long experience of working with side effects. They both became interested in the subject from working at the Swedish Medical Products Agency, something that later guided their residency decisions.
When asked about their spare time, Pär Hallberg says a lot of it is spent on work, but that he likes it so much that he does not mind the time it takes – and you can tell that Mia Wadelius and Pär Hallberg is dedicated to their work. When talking to them, they both constantly come with suggestions on how to improve the clinical work on side effects: Side effects should be part of the National Quality Registries, which are integrated into clinical workflows. Doctors should add blood samples with every side effect report. A government agency should be dedicated solely to side effects. And the list goes on.

They do, however, have some time outside of their offices. Mia Wadelius spends it with her children and grandchildren, learning French or working out at Friskis&Svettis – when she is not at her fisherman’s cottage by the Mediterranean Sea in the south of France, of course. Pär Hallberg on the other hand, travels to the world of books. He reads horror novels – the Stephen King kind – and has a, not so secret anymore, dream of being a fiction writer. He has actually written a book with a friend of his, a humorous tale about a godlike alien species with the goal of building a new planet.
“We think it’s great of course, and very funny, but it’s hard to get it published, the intrigue may be a bit too complex”, says Pär Hallberg.
Swedegene takes form
Most inherited risks of side effects are likely unknown and were even more so in 2009 when Pär Hallberg started to plan the Swedegene project, which is a collaboration between Uppsala University, Karolinska Institutet and the Swedish Medical Products Agency.
“This wasn’t my idea from the beginning. The idea to nationally collect knowledge of side effects and store them in a knowledge bank has been around for quite some time, but no one had the time to figure out how to actually do it. So I said I could do it. I put a plan together and started in small scale, and that was the start of Swedegene”, says Pär Hallberg.

Since the start, the project has collected samples from 3 000 patients from all over Sweden with 40 different side effects. It is a ton of work. Usually, a clinician discovers that a patient has an adverse reaction and reports this to the Swedish Medical Products Agency. The agency in turn hands it over to Swedegene along with a list of other recent cases. The reporting clinician is asked if it is OK to contact the patient, who is then asked to participate in the study.
“This is months or a year later, the patients aren’t hospitalized anymore when we get in contact with them”, explains Mia Wadelius.
The patient is interviewed over the phone about side effects, history of illness, and other personal information. A blood sample is then taken at a health centre close by and sent to the lab in Uppsala.
“It’s a simple principle, but it’s a long and laborious process and you need a lot of patience. What’s even harder is getting enough cases of the same side effect”, says Pär Hallberg.
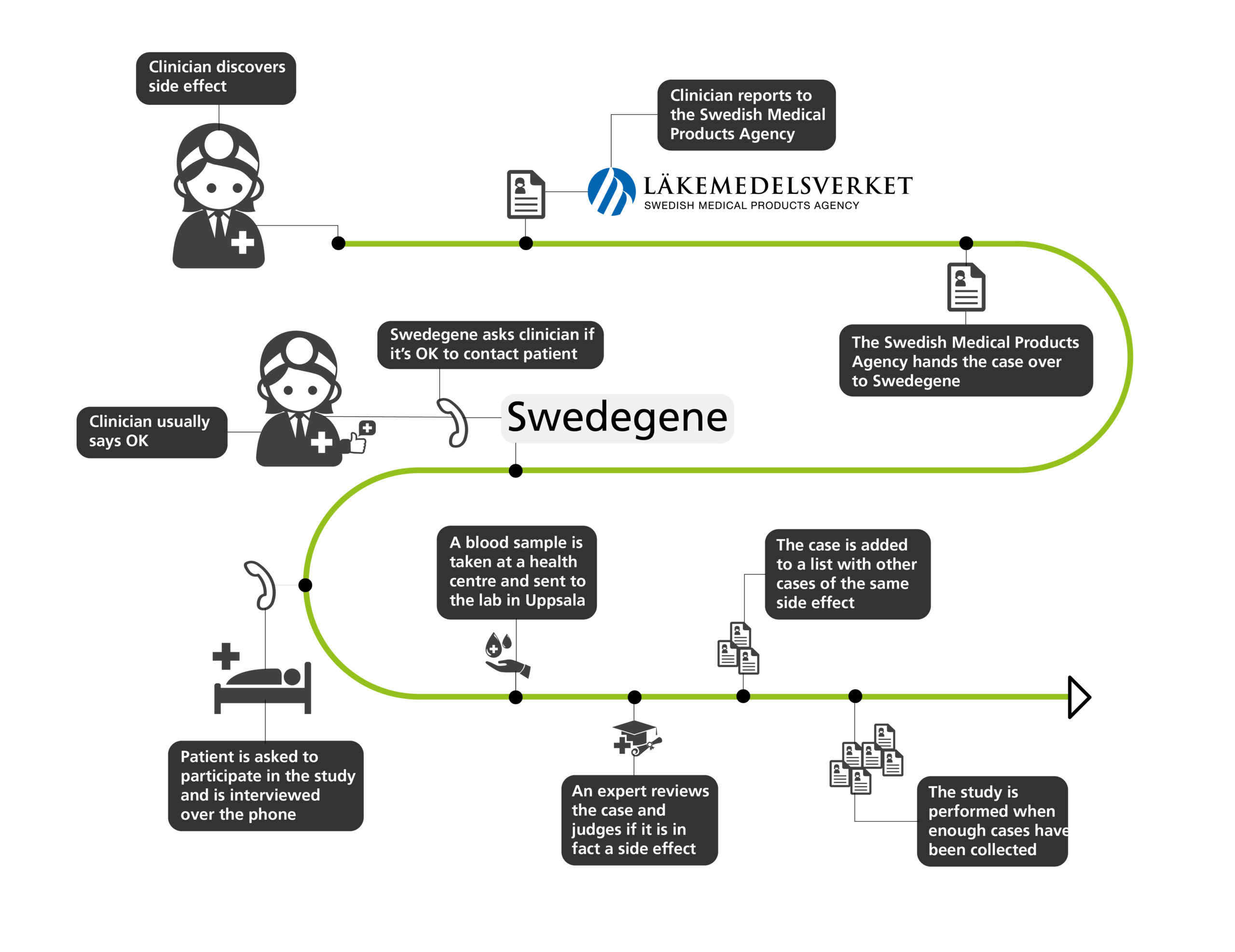
When enough samples have been collected the genetic analyses can begin – after an expert reviews the case and judges if it is in fact a side effect. If not, the patient is excluded from the study.
“It’s a good thing we have independent experts that assess causality, I would want to include all patients. For us, each patient is so valuable, it can take weeks to approach the person, do the interview and get the blood sample. It’s a lot of work, and it is frustrating when you need to exclude a person because of lack of evidence”, says Mia Wadelius.
Something that makes getting enough patients to study even harder is the fact that doctors do not always report the side effects to the Swedish Medical Products Agency, even though they are obliged by law to do so. About one percent of the severe side effects are reported.
“Likely a lot less for non-severe side effects. We thus miss 99 percent. I think the reason is lack of time, they choose not to report because of a heavy workload. I can understand that”, says Pär Hallberg.
 “I really should get rid of the folders”, says Mia Wadelius and adds that she has started to digitize, but that it takes a lot of time.
“I really should get rid of the folders”, says Mia Wadelius and adds that she has started to digitize, but that it takes a lot of time.
A slow moving machinery
In a perfect world, the results of this extensive research would be implemented in health care practices straight away. That is, unfortunately, not always the case.
Even where side effects are known and can be prevented, such as bone marrow impairment after taking the drug azathioprine, precautions are not always taken. A young man was for instance hospitalized because of this – he had mutations in both TPMT genes, and the enzyme with the same name could therefore not break down the toxic parts of the medicine. This led to bone marrow impairment, with an increased risk of infection and low levels of white blood cells that followed. This side effect was known and could have been foreseen with the help of genotyping – by examining his DNA and finding out that he had mutations on the genes.
“This is a real case that illustrates that even if these things are known, they are not implemented in health care yet”, says Pär Hallberg.
To be fair, this particular case has led to increased genotyping before prescribing azathioprine. It could, however, still be better. Genetic tests to detect possible side effects still lags behind.
“Take the recommendations for azathioprine for example. The information is there, but it’s a very weak suggestion of predicting risks with genetic tests, it’s not a strong recommendation that genotyping should be performed, and this is often the case. The technology is there, but it hasn’t been implemented in any wider scale so far”, says Pär Hallberg.

Economy is another issue when deciding whether to analyze these things in health care situations – it is not possible to genotype every single individual, even if potential side effects could be prevented. It simply costs too much, or is underprioritized.
“For the health care, we always have to motivate that we will break even. We need to show that doing this won’t just be a cost, but that there are financial incentives for doing these analyses clinically as well”, says Mia Wadelius.
Statistically, to find one case of the example above you would have to genotype about 200 patients.
“You have to calculate whether it’s financially beneficial to perform the analyses or if it’s cheaper to have the patient hospitalized for a month to treat the problem”, says Mia Wadelius.
Can’t you argue for the health benefits, despite the costs?
“That’s actually a hard thing to do. But that’s the reality we live in, if we had endless resources much of this would likely be implemented today, but we don’t, so you’ll have to choose the things you find most important and focus on those”, says Pär Hallberg.
“But of course”, Mia Wadelius adds, “If it’s a common problem and many people are affected by severe side effects that could be prevented with these tests, there’s a very big chance of the tests being offered. It’s not only about economy, it depends on how common the problem is”.

What makes this research even harder to implement is that even if a patient has been genotyped, the results risk being lost in the journal system used in Swedish health care. In some countries, the journal system alerts the doctor when trying to prescribe medicine if the patient has a genetic variant that causes an increased risk of side effects. This function does not exist in the Swedish journal system yet. In this system, tests results are also placed in chronological order with recent results appearing first in a long list.
“You won’t see these results after a month or so if the patient takes a lot of tests. After a few years you will definitely not see these test results if you don’t actively look for them. It would be good if the medical record had a specific tab for genotypes”, says Mia Wadelius.
Narcolepsy from vaccination
Something that likely could not have been prevented at the time being, according to Mia Wadelius and Pär Hallberg, is the narcolepsy caused by vaccination against the swine flu in the years 2009 and 2010. The narcolepsy caused was mainly the so called type 1, where those affected suffer from cataplexy – a tendency to suddenly experience muscle weakness and fall down when laughing or feeling excited. The person cannot move during this state, but can hear what is being said.
“After a finding like this, new treatment possibilities for narcolepsy could potentially be discovered”
Nation-wide vaccination campaigns were undertaken in for example Sweden and Finland, where large parts of the populations were vaccinated with the influenza vaccine Pandemrix. After the campaign, the narcolepsy incidence increased fifteen times in Sweden and Finland. A recent study from Swedegene shows that several genes increase the risk of developing narcolepsy after Pandemrix vaccination. To study this is strictly of academic interest, however, as the Pandemrix vaccine will not be used again, explains Mia Wadelius.
“You can also imagine that after a finding like this, new treatment possibilities for narcolepsy could potentially be discovered when you understand the mechanism behind narcolepsy development”, says Pär Hallberg.

So, would Pandemrix not have been used if the risks were known beforehand, if there were no alternative drugs? That’s not a simple question to answer, according to Pär Hallberg. The risk of getting narcolepsy was still very low, and considering what was at stake, the vaccine might actually have been used despite the risks.
“What was known at the time was that something very serious was on its way. Something that could potentially cause major catastrophes similar to the Spanish flu. That was the risk you were dealing with, so I can’t imagine anything else”, says Pär Hallberg.
Into the great unknown
The Swedegene project continues, but they are also part of a new project called Genomic Medicine Sweden – a massive national project recruiting 25 000 patients a year for different types of research. Mia Wadelius and Pär Hallberg, and their group, is, perhaps not too surprisingly, part of the pharmacogenomics and side effects part of the project – aiming to analyze four genes that affects eleven types of drugs.
The National Genomics Infrastructure (NGI) at SciLifeLab helps these projects with sequence analyses. Previously, parts of the genome has been sequenced, but from now on, whole genome sequencing will be performed. This means a lot more data, and more complex analyses. For this reason, they have hired a bioinformatician, Joel Ås, who with the help of SciLifeLab has developed a new system, and help make sense of the results.

There is a lot more to be discovered when it comes to side effects. What that is exactly, and to what extent the results will be implemented into health care routines, is anyone’s guess – but Mia Wadelius and Pär Hallberg are not discouraged.
“As long as we keep on doing this research and can show that genotyping prevents side effects, then we can hopefully raise some awareness and eventually see some change”, says Pär Hallberg.