Cecilia Williams
KTH Royal Institute of Technology (Professor), Karolinska Institutet (Visiting Professor), University of Houston (adjunct faculty)
Research Interests
Some cancers are more common in men, and others are more common in women. Some are related to sex hormones, sometimes in unexpected ways such as that estrogen protects against colorectal cancer. How and why does it do this? While simultaneously increasing the growth of breast cancer? And how is ovarian cancer affected, especially the subtype Granulosa Cell Tumor?
Estrogenic signaling is mediated by the estrogen receptors ERalpha, ERbeta, and GPER1. ERalpha and ERbeta are ligand-activated nuclear receptors and excellent therapeutic targets. Inactivation of ERalpha is for example the most successful breast cancer treatment to date. However, treatment resistance frequently develops, and we need to understand the detailed molecular mechanisms and how this can be countered.
The Williams’ group focuses on understanding key molecular mechanisms in cancer, combining large-scale omics together with focused mechanistic experiments and in vivo studies. Our goal is to understand critical cancer pathways linked to estrogen signaling so that we can suggest sex-tailored cancer treatments and preventive approaches, as well as biomarkers, to be developed for clinical use.
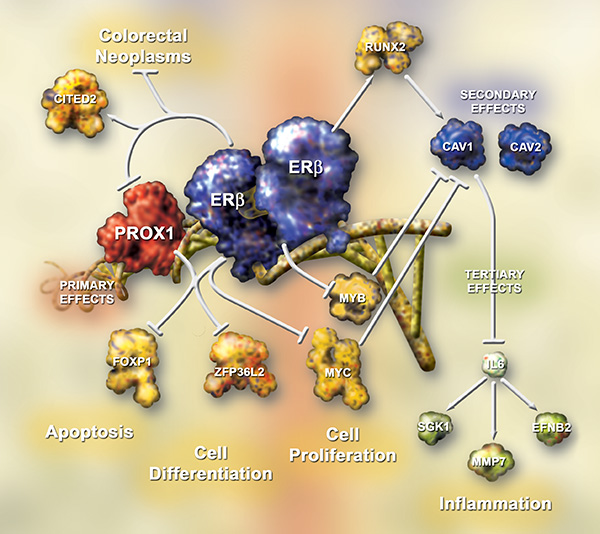
Our previous work includes the identification of the estrogen-regulated transcriptome, the roles of specific microRNAs in cell migration, and expression and regulations of lncRNAs, with a focus on cancer. With cell and animal models, we have demonstrated sex differences in colorectal cancer initiation and progression. Combining transcriptomic analysis of human biopsies and machine learning, we have identified that prognostic biomarker candidates for colorectal cancer depends on sex. We have also shown that ERbeta attenuates colitis-induced colorectal cancer via crosstalk with several transcription factors and can impact gut microbiota in a sex-dependent manner. Currently, we are exploring the effect of estrogen signaling via ERbeta on tumor microenvironment in colorectal cancer and ovarian granulosa cell tumor using cutting-edge technologies such as ChIP-seq, scRNA-seq, spatial proteomic and spatial transcriptomic. Our studies can help generate better therapeutics against cancer, including its prevention.

Group members
- Cecilia Williams, Professor and Group leader
- Amena Archer, Lab manager
- Matilda Holm, Post doc
- Fatima Liliana Monteiro, Postdoc
- Madeleine Birgersson, Ph.D.
- Lina Stepanauskaite, Ph.D. student
- Linnéa Lindquist, Ph.D. student
- Udari Jayathilake, Master student
- Elin Lindbom, Master student
- Susanna Hellberg, Master student
Contact
cecilia.williams@scilifelab.se