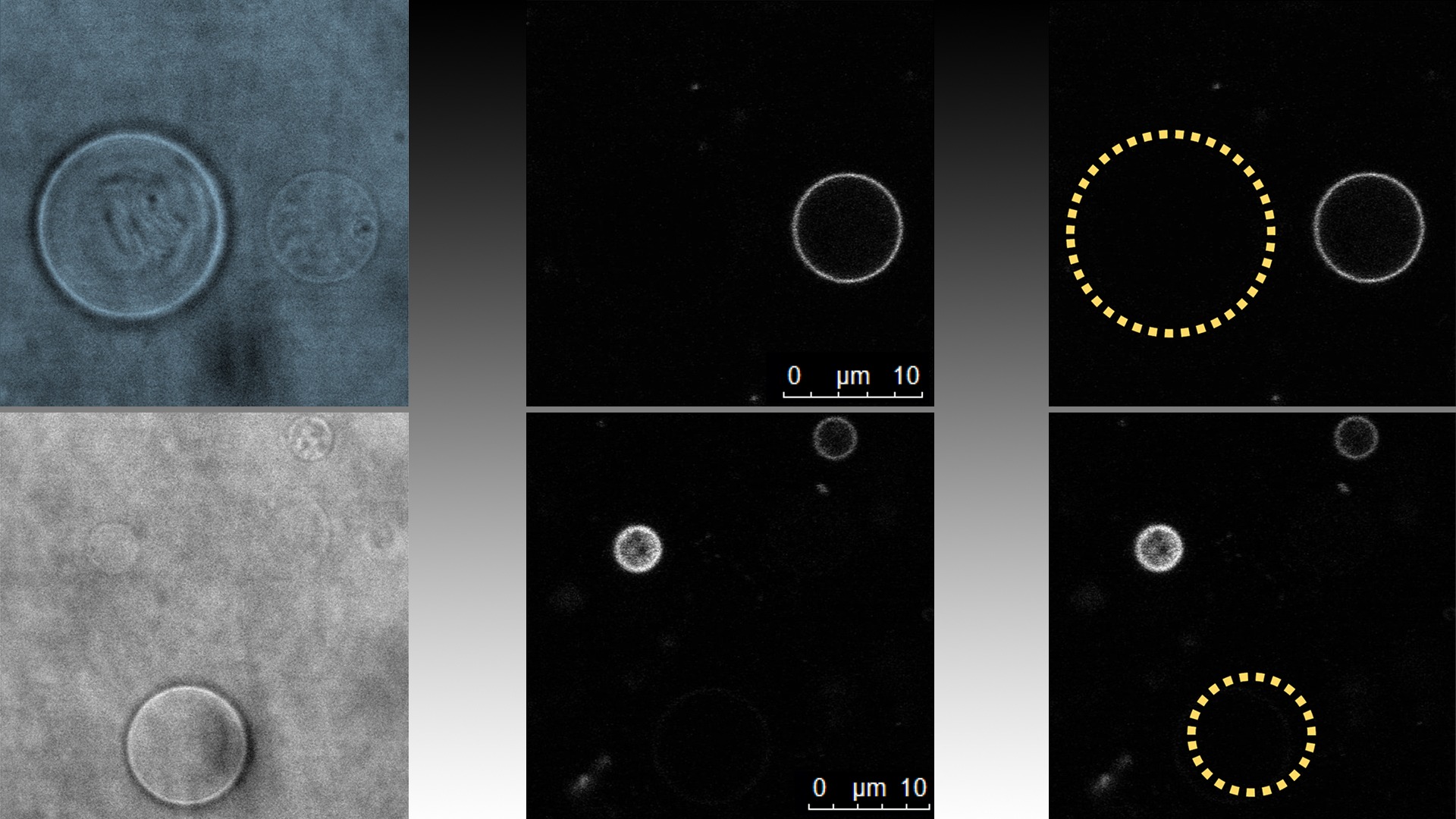
Cooperativity of α-synuclein binding to lipid membranes
Alpha-synuclein is a neuronal protein whose aggregation is associated with Parkinson’s disease, but its healthy function is not well understood. Assisted by the Advanced Light Microscopy (ALM) node at the SciLifeLab IMT unit, researchers from Emma Sparr and Sara Linse’s group at Lund University, have discovered that α-synuclein (αS) binding to lipid membranes is cooperative which may have implications for the biological function of the protein. The study was published in ACS Chemical Neuroscience.
Fluorescence correlation spectroscopy, confocal microscopy, and cryo-TEM results showed that in the excess of vesicles, αS does not distribute randomly but binds only to a fraction of all available vesicles. This means that the affinity of the protein to a membrane is higher where there is already protein bound compared to a bare membrane.
“The phenomenon may have implications for αS function in synaptic transmission and other membrane remodeling events, since these processes are known to be controlled by proteins that self-assemble on the membrane”, says Katarzyna Makasewicz (Lund University), first author of the publication.
Due to the strong binding of αS to the vesicles the researchers had to come up with a new approach to extract the information of interest from the FCS data.
“A difficulty during the study was that the fluorescence from the vesicles was so intense, due to the large amount of dye-tagged αS bound to them, that unbound αS, which are also present, could not be distinguished by the conventional approaches used with the FCS technique. Eventually we came up with a new approach to treat the FCS data which allowed us to estimate the number of αS bound to each vesicle as well as the amount of unbound αS”, says Stefan Wennmalm (SciLifeLab/KTH), staff scientist at the Advanced Light Microscopy node.