Functional precision medicine used to assign tailored therapies to leukemia patients
Co-led by the SciLifeLab Director Olli Kallioniemi (Karolinska Institutet), researchers from the Institute of Molecular Medicine Finland (FIMM) at the University of Helsinki and Helsinki University hospital, Karolinska Institutet in Solna, Sweden and Haukeland University Hospital in Bergen, Norway, have developed and tested a functional precision approach to assign tailored therapies to patients diagnosed with hematological cancers.
The study, which spans across ten years, shows that a treatment selection based on individual drug sensitivity testing can be clinically useful in patients with advanced and relapsed acute myeloid leukemia (AML), a cancer where conventional therapies have a limited effect.
The researchers utilized a novel functional and molecular tumor board (FMTB) strategy, in which the patient’s genomic and molecular profiling data was integrated with high-throughput drug testing data to determine the best possible treatment for each individual patient, in real time. Their findings were published in Cancer Discovery, a flagship journal of the American Association for Cancer Research.
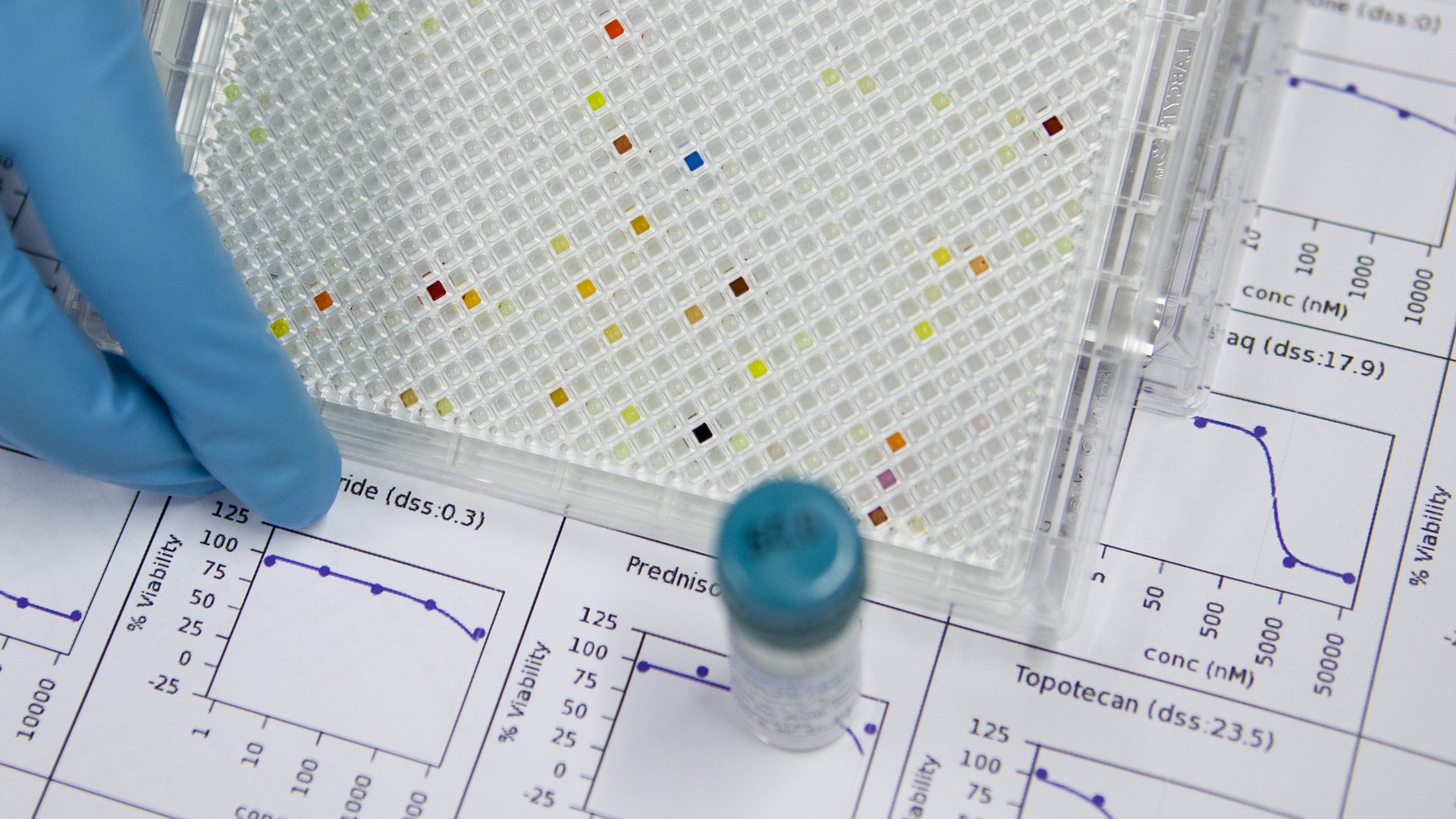
The effect of more than 500 drugs were tested on cells from 186 patients with AML. The tested compounds included 180 approved cancer drugs and many emerging or investigational cancer drugs.
“Our approach combines rich molecular profiling data with comprehensive drug sensitivity testing of patient cells to advance the therapy decision-making system for the leukemia patients,” says first author Disha Malani, who completed her PhD studies while working on the project, in a press release from FIMM.
The new approach helped to identify actionable treatments for almost all (98 percent) of the ALM patients, which is exceptional compared to conventional genomics-based precision medicine approaches. 37 adult relapsed AML patients were then selected, treated with the recommended drugs and included in a clinical study.
For the patients, most of whom had no other available treatment options, the outcome was beyond expectations, with an overall clinical response rate of 59 percent. Additionally, five patients were successfully bridged to curative hematopoietic stem cell transplantation therapy and many of these patients are now in long-term remission.
“Compared to genomics-based precision medicine, drug testing provides informative results in a substantially higher fraction of patients as well as for more drugs. We also developed a new method of communicating results to the clinic, via a functional and molecular tumor board, something that we believe can in the future grow to become a system that learns from past cases to provide increasingly better predictions for the patient treatment” says Kimmo Porkka, from the Helsinki University Hospital Comprehensive Cancer Center and a co-leader of the study.
The drug testing technology only takes three days and provides clinically actionable data faster than classic genomic profiling. This is important in a medical emergency like AML, where treatments often need to start right away.
“This approach is advantageous in rapidly recommending treatments for patients for whom standard therapy alternatives have been exhausted and alternative therapy options are urgently needed”, says Kimmo Porkka.
By comparing the molecular profiling data from the patients with the response of each one of the 500 drugs, the researchers could identify the molecular factors behind the drug efficacy of each drug. This made it possible to identify several new biomarkers for drug response, such as the overexpression of the IL15 gene, which is functionally and mechanistically associated with resistance to FLT3-inhibitor treatments.
“With the clinical introduction of functional precision medicine, we can expect faster and broader implementation of precision medicine across different cancer types. We still need further work in standardizing the methods, getting access to more drugs for patient treatment and data from randomized clinical trials, something that we wish to achieve in collaboration with the global research community, hospitals and the pharma industry”, says Olli Kallioniemi, who co-led the study.
“The same strategy is relevant to SciLifeLab with the launch of the precision medicine capability and the DDLS program. For example, over 150 Swedish AML samples (and many additional cancer types) have in recent years undergone deep multi-level molecular profiling and functional drug testing, with the first clinical trials being planned”, he continues.
Read the press release from FIMM
Photo: FIMM / University of Helsinki