Nerve signals may hold key to understand type 1 diabetes
Nerve signals can influence the immune cells that target and destroy insulin-producing cells in type 1 diabetes. This according to a recent international study co-led by SciLifeLab Fellow Gustaf Christoffersson (UU) involving researchers from Uppsala Universitet and the La Jolla Institute for Immunology, USA. These findings may be used to further explain how the immune system is regulated and could be used to explore novel treatments for the disease.
The pancreas, a small sweet potato-shaped organ behind the stomach, is studded with small cell clusters that house insulin-producing beta cells. In people with type 1 diabetes, the beta cells are attacked by the body’s own immune system – and the exact cause for this still remains unknown.
So far, scientists have been able to observe a pattern of beta-cell death, as some cells are killed off in big patches, while others remain untouched by the immune cells. This led the researchers to look for clues as to why some are ignored while others are not.
In the study, co-led by SciLifeLab Fellow Gustaf Christoffersson (UU), and published in Science Advances, the researchers were able to establish a link between the nervous system and this patchy cell death. Their new findings in a mouse model suggest that blocking nerve signals to the pancreas could stop patients from ever developing type 1 diabetes.
“It’s astonishing that this process may be stoppable through neuronal influence,” says Gustaf Christoffersson, first author of the study.
Despite there being both environmental and genetic risk factors involved, type 1 diabetes often seemingly strikes at random. One theory has been that these patches have differences in blood flow or they have been damaged by a virus that might be sparking an immune attack.
Recently, however, researchers have been exploring a new field called neuroimmunology. The idea is that nerve signals can affect immune cells and if so, could these nerve signals drive immune cells to attack certain areas of the pancreas?
“We thought that could explain a lot,” says Christoffersson.
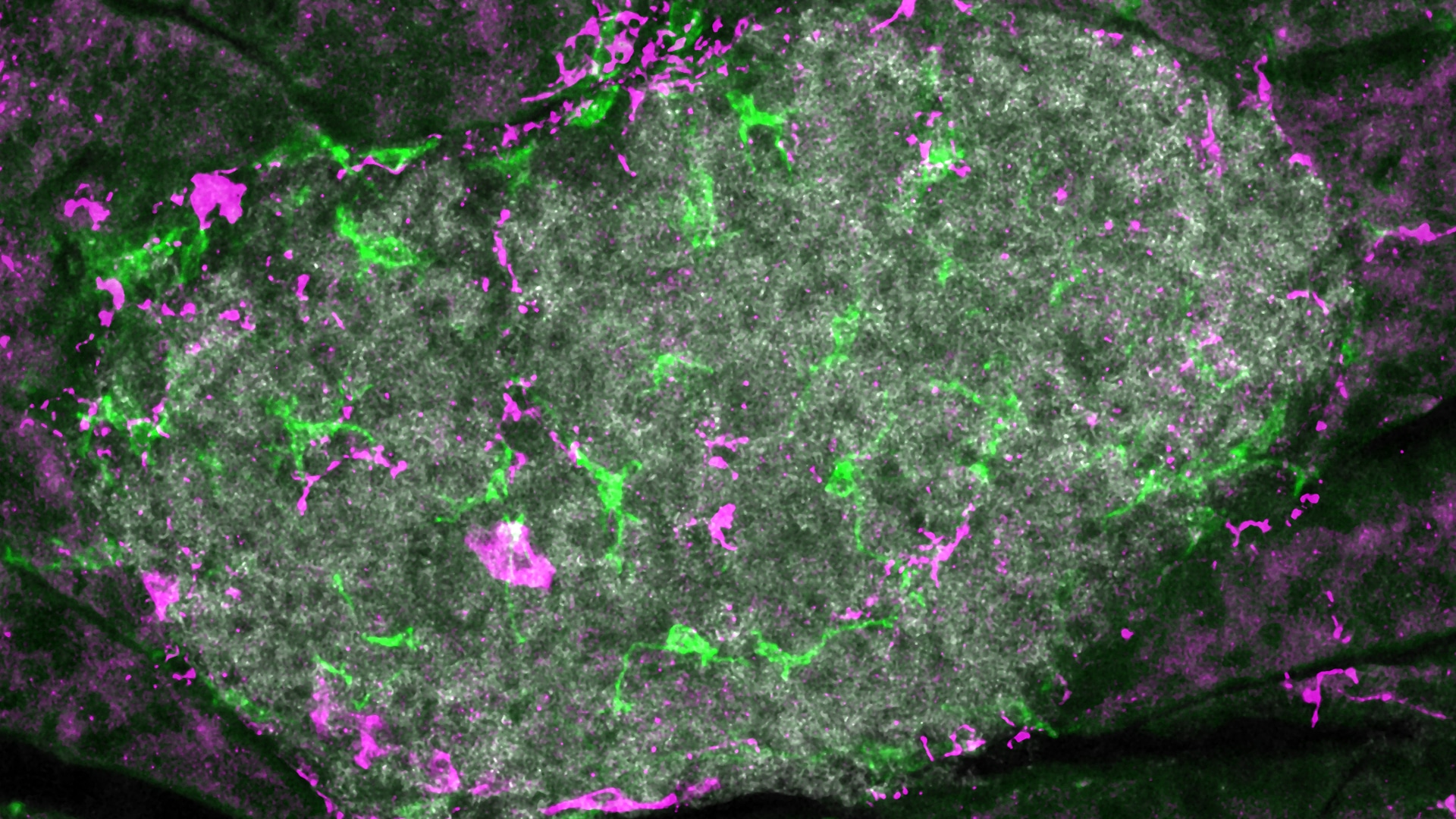
The researchers tested this theory by using a mouse model that can be experimentally induced to have beta-cell death. To block most of the sympathetic nerve signals to the pancreas they “denervated” the pancreas in the mice, either surgically or through the use of a neurotoxin or a pharmacological agent. They then tracked the movements of immune cells around the beta cells using advanced in vivo imaging.
The team found that blocking the nerve signals protected mice from beta-cell death.
“We were pretty surprised to see that these nerve blockers led to pretty significant differences in the onset of diabetes,” says Christoffersson.
”More work needs to be done before this method can be tested on people”, says Matthias von Herrath,one of the study’s senior authors.
”Doctors would first need a reliable way to identify patients at risk of type 1 diabetes onset. Once these patients are identified, they could be treated with electrostimulation or drugs to block nerve signals”, von Herrath continues.
The researchers believe that this discovery has the potential to explain more than just the “patchiness” seen in type 1 diabetes. Because the same “patchiness” is seen in several autoimmune diseases, but in a symmetrical pattern. One example is the skin condition vitiligo which causes the skin to lose pigmentation in symmetrical areas across the faces and hands. Arthritis also tends to strike symmetrically, with inflammation in both knees, elbows, or wrist joints.
The new study suggests that these areas may be innervated by nerves that branch out symmetrically through the body.
“This symmetry is very striking, and it’s been almost impossible to explain”, says von Herrath.
Christoffersson and von Herrath both believe that breakthroughs in neuroimmunology could have broad implications for explaining why the body turns against its own organs in many autoimmune diseases. Going forward, they also hope to investigate the cellular mechanisms that connect the nervous system and type 1 diabetes in humans.
The researchers now hope that breakthroughs in neuroimmunology could play an important role when trying to find out why the body turns against its own organs in many autoimmune diseases. Looking forward, they plan to investigate the cellular mechanisms behind the nervous system and type-1 diabetes connection in humans.