Novel mechanism for manipulating ion channels discovered
Facilitated by the ScilLifeLab platform Chemical Biology Consortium Sweden (CBCS), researchers from Linköping University (LiU) took a closer look at 10 000 molecules from the “SciLifeLab Compound Collection” using high throughput screening technology.
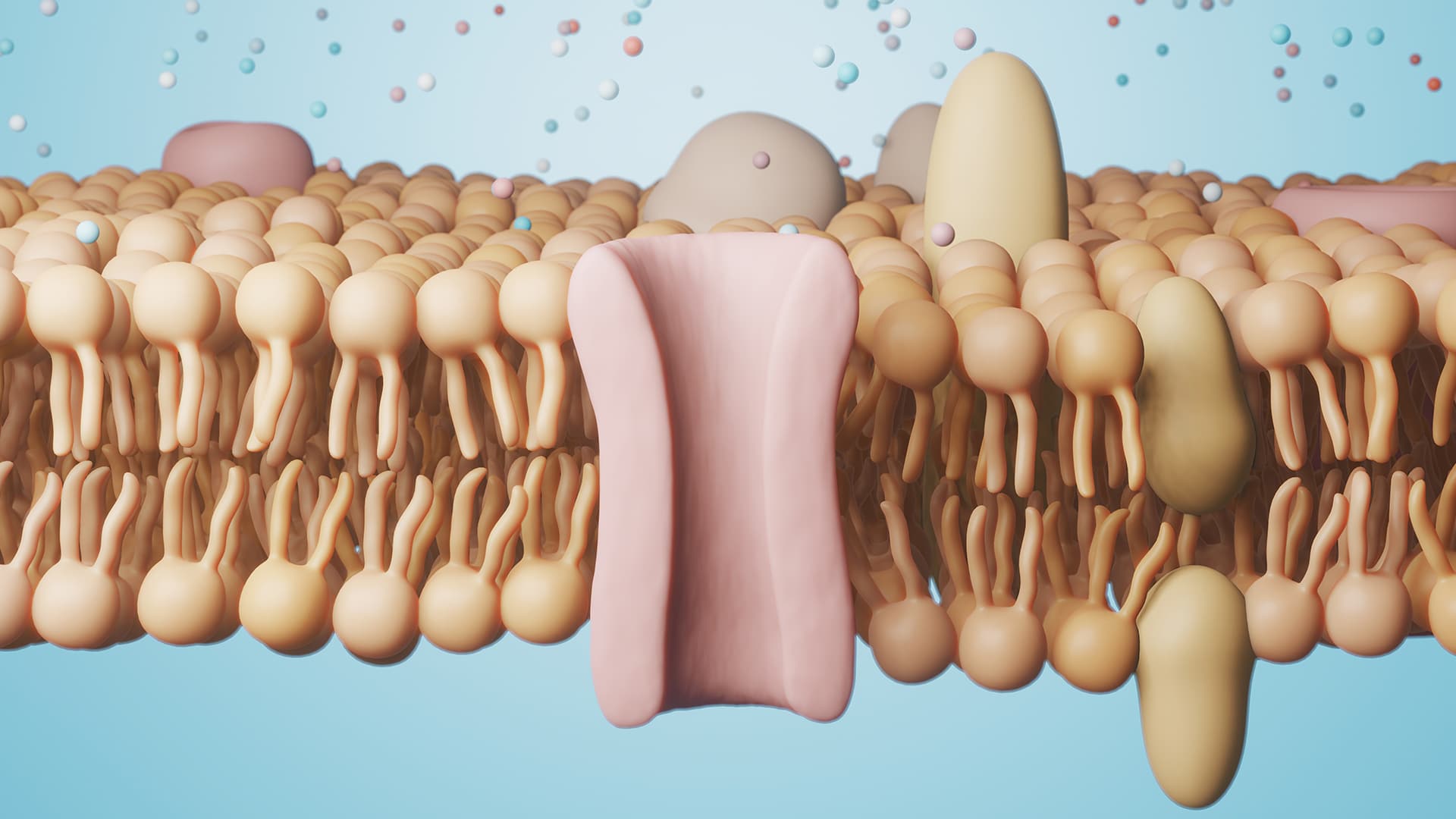
Certain types of drugs can either open or close ion channels, blocking or increasing nerve signaling in the nervous system. Blocking certain ion channels can for example lead to a reduced experience of pain locally. While much is known about substances that close ion channels, the opposite is true for substances that can cause them to open. And treatments for conditions such as epilepsy, chronic pain, and heart arrhythmias are less than optimal.
The LiU-researchers wished to identify molecules with well-defined effects on different types of ion channels and subsequently design molecules based on those effects.
Using a specially designed ion channel the researchers tested 10 000 substances from the CBCS, which houses over 200 000 molecules in their Compound Collection. Of the 10 000 tested substances, 247 stood out for having very similar structures. And the similarity closely resembled that of the anticoagulant drug warfarin.
“High-throughput screening technology has long been used in the pharmaceutical industry, but it’s quite new in academic research. The pharmaceutical industry starts with an extremely specific question and is looking for a substance that can act against a particular disease. In contrast, we used the technology to seek answers to an extremely open question, and were surprised by the answers”, says last author Fredrik Elinder (Linköping University) in the LiU press release.
The ion channel had earlier been used to study resin acids which keep the channel in its open state. Now they hoped to be able to discover substances that act upon the channel in the same way as the resin. The substances that they found, however, acted by a different mechanism altogether.
“We found that the new class of substances, with warfarin-like structures, bind to a completely different place on the ion channel: they act on the side of the ion channel that is inside the cell, where the coupling between the two parts of the ion channel is located. They keep this coupling in its active position which in turn keeps the channel open. This was a huge surprise for us. As far as I know, no other substance has been shown to act on the coupling between the voltage sensors and the pore”, says Fredrik Elinder.
Read the entire press release from Linköping University here