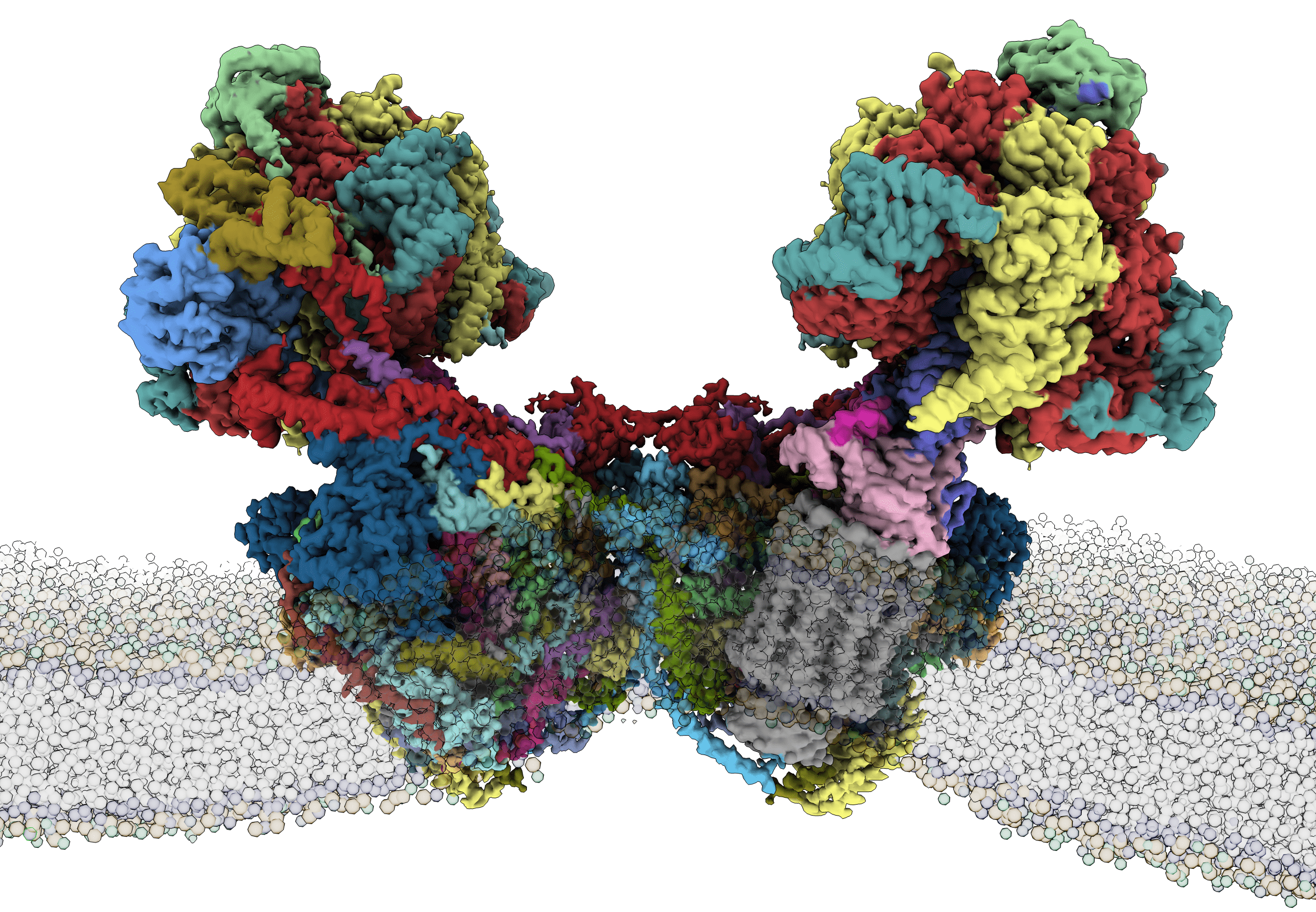
Structure of a mitochondrial ATP synthase
SciLifeLab researchers Alexander Mühleip (Stockholm University) and Alexey Amunts (Stockholm University) solved the structure of a mitochondrial ATP synthase with native lipids.
ATP synthase is a universal molecular machine for energy conversion. By coupling to cellular respiration in mitochondria, it catalyzes conversion of chemical energy of cells.
Mitochondrial ATP synthase is composed of dimers that, when come together, form membrane curvature that is essential for efficient energy conversion. While the mitochondrial signature lipid cardiolipin and its interactions with proteins are believed to contribute to this process, it was not directly visualized before. In addition, it was unclear to what extent the ATP synthase has diverged across different species.
Researcher Alexander Mühleip, from Amunts lab, used the single-cell photosynthetic organism Euglena gracilis, which belongs to a phylum that also includes human parasites, to extract the mitochondrial ATP synthase. Its structure was then determined using cryo-EM, allowing the reconstruction of the atomic model. The high resolution of the cryo-EM density map allowed identification of 29 different protein subunits and 25 cardiolipin molecules. Some of the cardiolipins appear to modulate the critical channel for proton transfer that fuels the machine, which is the first evidence for their direct involvement.
The model shows that the Euglena mitochondrial ATP synthase is highly divergent with 13 protein subunits being specific to this system. The new subunits contribute to the formation of dimers glued by lipids, providing experimental evidence that the key functional property of membrane curvature induction has likely occurred independently in evolution. Furthermore, a membrane-embedded subcomplex was found at the periphery of the ATP synthase, displacing the membrane. Sarah McComas (Stockholm University), performed molecular dynamics simulations supporting that the newly found subcomplex contributes to the membrane curvature.
The atomic model of the mitochondrial ATP synthase with native lipids provides a new perspective for its functional analysis. The different type of molecular organization of this essential energy production machinery re-establishes the defining features of the complex and opens the door for reconstructing the evolution of the mitochondrial ATP synthase. Finally, identified similarities to the human parasites provide a new perspective for a therapeutic exploration.
More information:
Structure of a mitochondrial ATP synthase with bound native cardiolipin. Muhleip A., McComas S. & Amunts A. eLife 8:e51179