Target Product Profiling & Drug Safety Assessment
This unit gives support to principal investigators and innovation agencies in preparing a first target product profile, assists with platform and project coordination, and gives assessment of target dependent toxicity and support with the design of pharmacological studies in vivo.
Target Product Profiling
The concept of Target Product Profiling (TPP) was introduced by the USA Food and Drug Administration (FDA) in 2007. The purpose of a TPP is to provide a format for describing a drug project that can be used throughout the drug development process in discussions with reviewing bodies and investors. Directors Per Arvidsson and Kristian Sandberg work closely together with principal investigators (PIs) and Innovation Agencies to prepare a preProject report (the first draft of a TPP) when a new project enters the Drug Discovery and Development platform at SciLifeLab. The following dimensions of a drug discovery project are described in the preProject report:
- Scientific validity of the therapeutic approach
- Medical need and differentiation from Standard of Care
- Safety concerns
- Competitive landscape
- Patent and publication strategy
- Feasibility of conducting a Phase II study
- Competence and ability of the PI team
- Technical feasibility to develop a drug
- PI entrepreneurship
Drug Safety Assessment
The DDD platform at SciLifeLab has a strategic collaboration with RISE for safety assessment of drug projects in the platform. Every project is evaluated for potential target safety concerns, e.g. toxicity due to agonism or inhibition of the target protein. This analysis may give information vital for the success of the project, and can even help at an early stage to eliminate non-druggable targets.
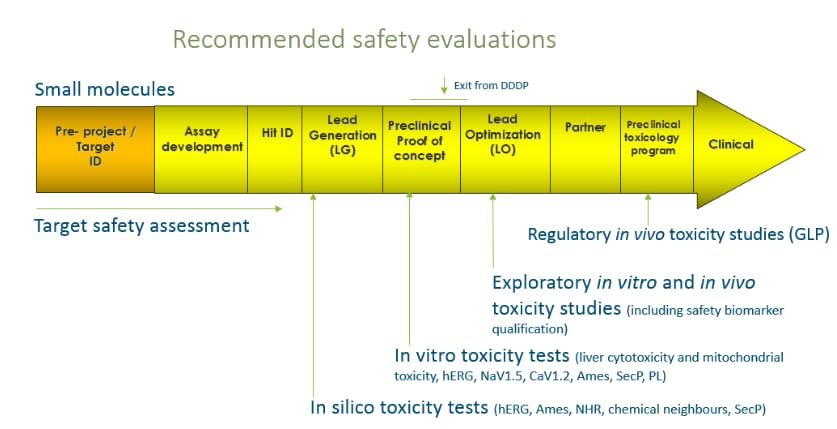
For small molecules, chemical toxicity properties are screened in the DDD process (see figure). Safety support to antibody projects focuses around avoidance of cytokine storms, prediction of immunogenicity and presence of anti-drug antibodies.
Design of conclusive In Vivo studies
Preclinical proof of concept studies are essential to demonstrate feasibility for further development of lead compounds and antibodies. Our project coordinator Rebecka Klintenberg has extensive experience in pharmacological studies and leads a group with the task to give advise to the principal investigator. Key questions discussed are:
- Animal model and power of study, including ethical aspects for animal welfare
- Administration of compound and antibody
- How to sample for pharmacokinetic and pharmacodynamic studies
- Dosing of compound or antibody
- Determination of in vivo exposure of compound or antibody
- How to demonstrate efficacy on biomarkers
- Determination of therapeutic window
